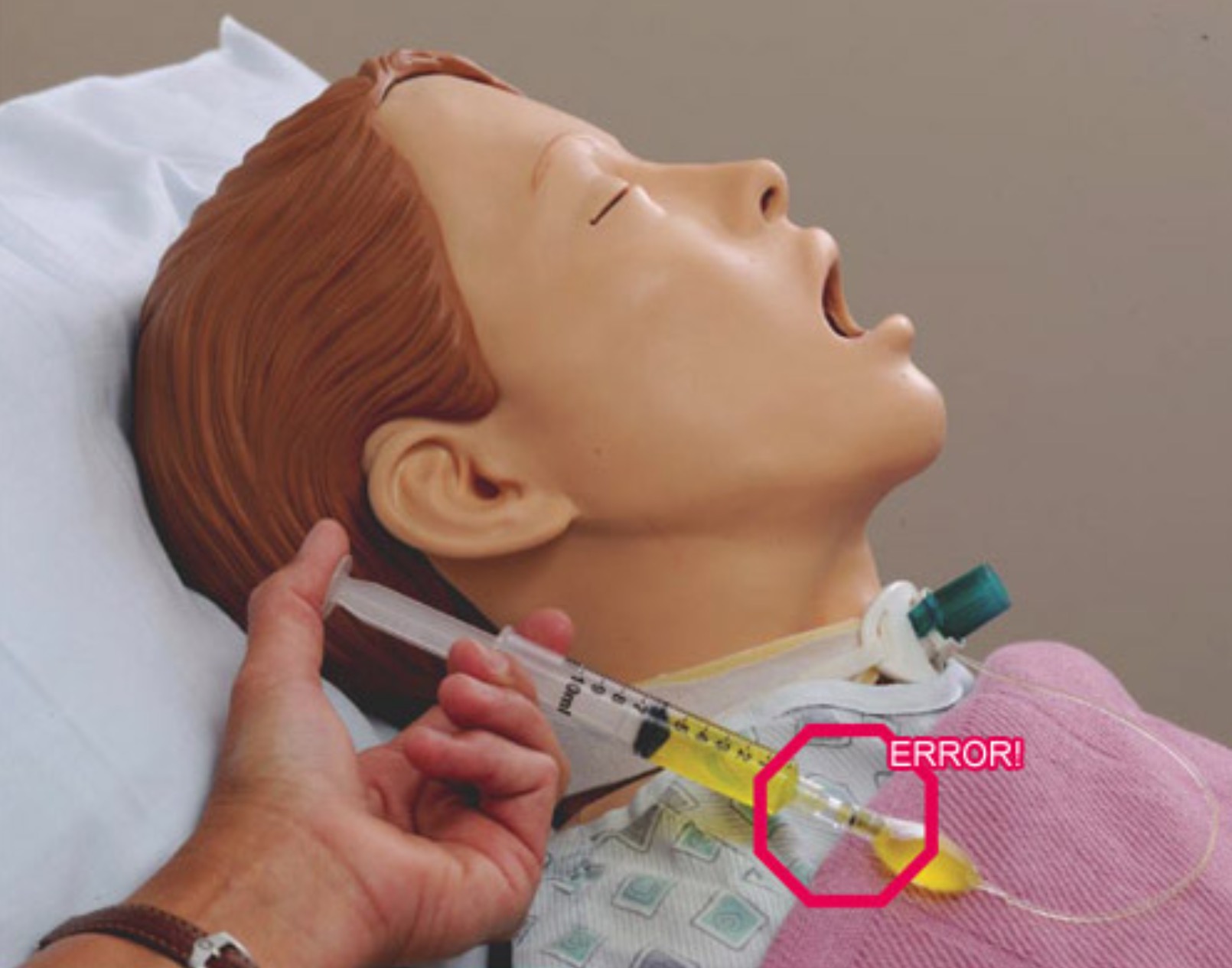
 Recently a tragic mix up where oxygen tubing was connected to a urinary catheter resulted in the death of ex-Socceroo Steve Herczeg (see here).
Recently a tragic mix up where oxygen tubing was connected to a urinary catheter resulted in the death of ex-Socceroo Steve Herczeg (see here).
‘How can anyone make this mistake?’
Unfortunately events like this occur regularly – we often only hear of them via the media – our error report systems lacking transparency (see here).
This link from the FDA (see here) documents numerous similar mis-connections.
We are all human and we will always make mistakes. Those who build a safety system that depends on an absence of human mistakes will fail utterly.
We can and should design our equipment based on an international standard to reduce the likelihood of misconnection.
The International Organisation for Standardization recently released a set of standards for connectors (see here and see here)- we must ensure that this is introduced worldwide as soon as possible.
International standards and colour coding has been implemented for enteral feeding devices (see here, and here) in some countries but not others. We welcome and support their universal adoption.
In March 2017 the ACQSHC released a joint statement on introducing standard connectors for neuraxial medication (see here). We are unsure of a timeframe for implementation.
In regards to the Steve Herczeg oxygen tubing to urinary catheter case a common question is how could this physically have occurred? Oxygen tubing and urinary catheters both have ‘female’ ends so they should not connect.
‘Male – male’ connectors exist throughout hospitals to allow one piece of oxygen tubing to be attached to another. This could have been an issue in the Steve Herczeg case allowing the oxygen tubing to connect to the urinary catheter. This could also be something a patient does to themselves noticing 2 ends of tubing which appear to have detached and attaching them back together.



6 Comments