This is a letter we wish the TGA will write:
The TGA has made the decision to ban indistinct pourable chlorhexidine solutions.
This decision is in keeping with recommendations from the root cause analysis (RCA) performed after the accidental epidural injection of chlorhexidine at St George Hospital in 2010. The RCA stated ‘all chlorhexidine solutions should be coloured in a way which clearly distinguishes them from any fluid for injection’.
The St George Hospital incident is not an isolated event. We acknowledge there was a profound near miss event in the weeks prior. Also in 2004 Mary McLinton had chlorhexidine injected into the blood supply to her brain and died two weeks later. ‘Gina’s Story’ portrays how arterial injection resulted in leg amputation at the waist. We are aware of many other accidental administration and near miss events which have occurred within Australia and worldwide. We recognise there is likely to be many other cases contained within error reporting systems.
To our knowledge accidental administration of chlorhexidine has only been reported in the presence of indistinct solutions. There is no benefit in having pourable indistinct chlorhexidine in healthcare institutions and while it exists it represents a serious unnecessary risk to patient safety.
We acknowledge correspondence from the Australian Commission on Safety and Quality in Healthcare that we work with suppliers to ensure no chlorhexidine is of pale colour, and alerts from the Australian and New Zealand College of Anaesthetists in response to another case reported within the last few months that ‘only highly tinted skin preparations should be used‘.
We commend those companies who have already changed the colouring of their chlorhexidine and alcohol solutions. We request that those who haven’t do so. In the interim we will ensure that indistinct chlorhexidine and alcohol is removed from healthcare institutions.
We recognise the complexity of healthcare environments. Some hospitals which had previously replaced indistinct chlorhexidine with vivid solutions have gone back to ordering indistinct chlorhexidine for no other reason than procurement staff being unaware of the issue.
We accept, given our position of protecting the health and safety of Australians, the most effective way to remove this unnecessary hazard is to ban it from healthcare institutions. Further we will contact our counterparts elsewhere in the world (FDA, MHRA, others), inform them of our decision and encourage them to do the same for the safety of patients worldwide.
We recognise this issue reveals inefficiencies with existing safety improvement systems. There is a deficiency with error reporting both in Australia and worldwide. Each state and each individual private hospital have their own siloed reporting systems which lack transparency. There is minimal communication between these error reporting systems. Many of these reporting systems need to be updated to allow a ‘search’ function allowing better capture of clustered recurring events.
We accept there is minimal awareness by front line healthcare staff of our own reporting system ‘IRIS’ and that there needs to be direct communication with IRIS and all other healthcare error reporting systems in Australia.
Further we acknowledge the need for a transparent hazard reporting system. Many frontline staff recognised the risk of indistinct chlorhexidine before the St George incident yet without a system to report this hazard little could be done by them to remove it.
We accept it is unsatisfactory that 500 healthcare staff have felt the need to resort to a petition to remove an unnecessary hazard from the front line.
We expect to see huge improvements in error and hazard reporting when we have efficient, transparent systems in place which allow front line staff to observe effective interventions being delivered in response to their reports.
We will clearly communicate how and when our hazard and error reporting systems are being improved.
Australia has a healthcare system which is renowned worldwide. We believe in acknowledging and rectifying these issues we position Australia as a world leader in healthcare safety.
Please feel free to contact us directly if you have any questions regarding this matter.
Thank you.
The TGA haven’t written this letter yet – please support this petition and provide them with the encouragement they need.
This issue highlights the failure of hierarchical top down structures in allowing work environments to be improved for patient safety.
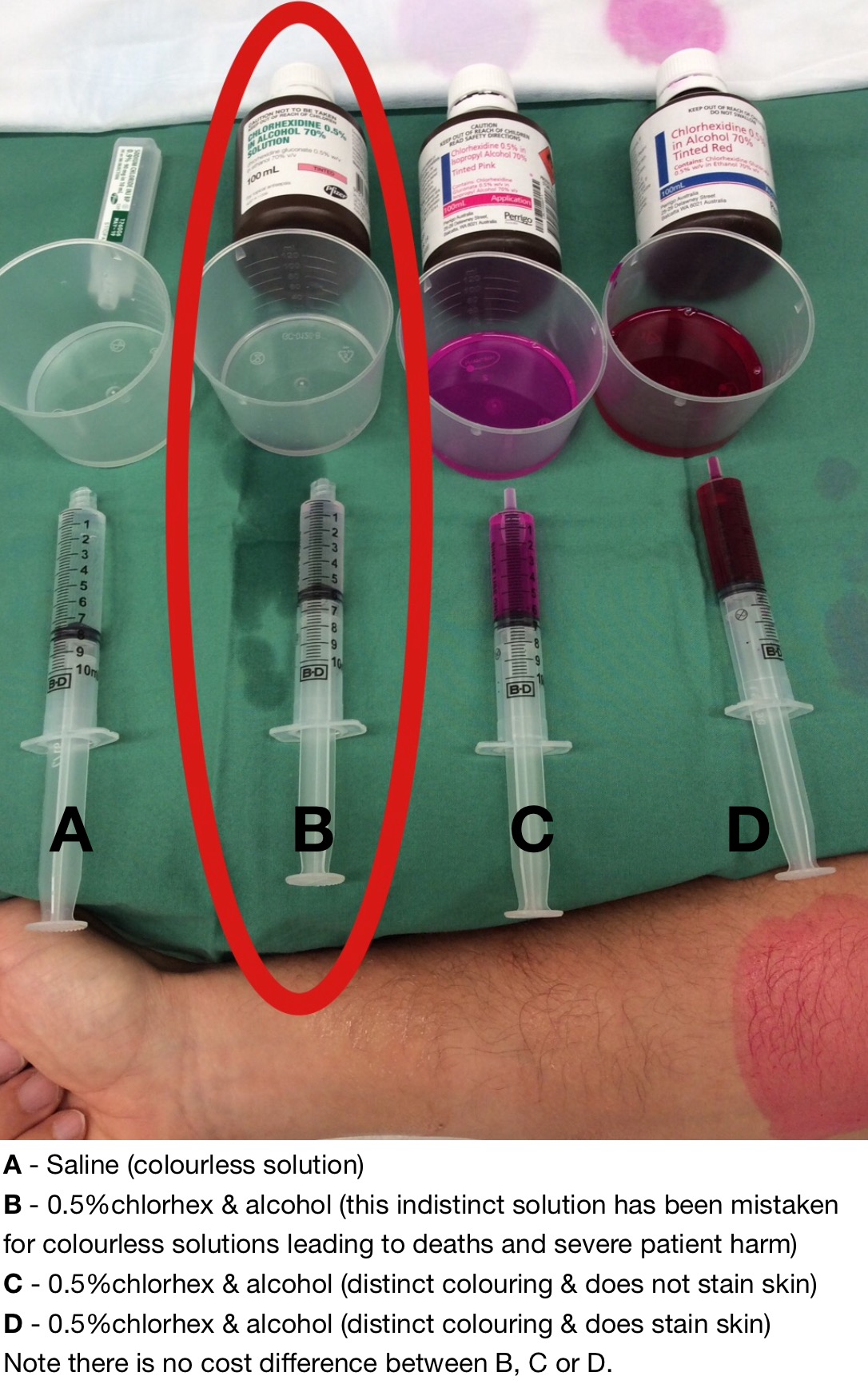
In the meantime there is an avenue for you to have it removed from your hospital. One of the manufacturers – Perrigo / Orion – has listened to our concerns and have gone to great lengths to produce 0.5% chlorhexidine in alcohol which is distinct. Their tinted red (D) stains the skin, while their tinted pink (C) does not stain the skin.

If you work in a facility which has indistinct chlorhexidine then please pass this post on to your hospital procurement officers & department heads.
Please note we have no financial interest with Perrigo / Orion or any of the other products discussed on the PatientSafe Network.
Contact details for Perrigo Australia:
PH 1800 805546
Email – customerservice@perrigo.com.au

One Comment