
Ruby Yan Chen, a 3 year old from Queensland, died from an avoidable air embolus (see here).
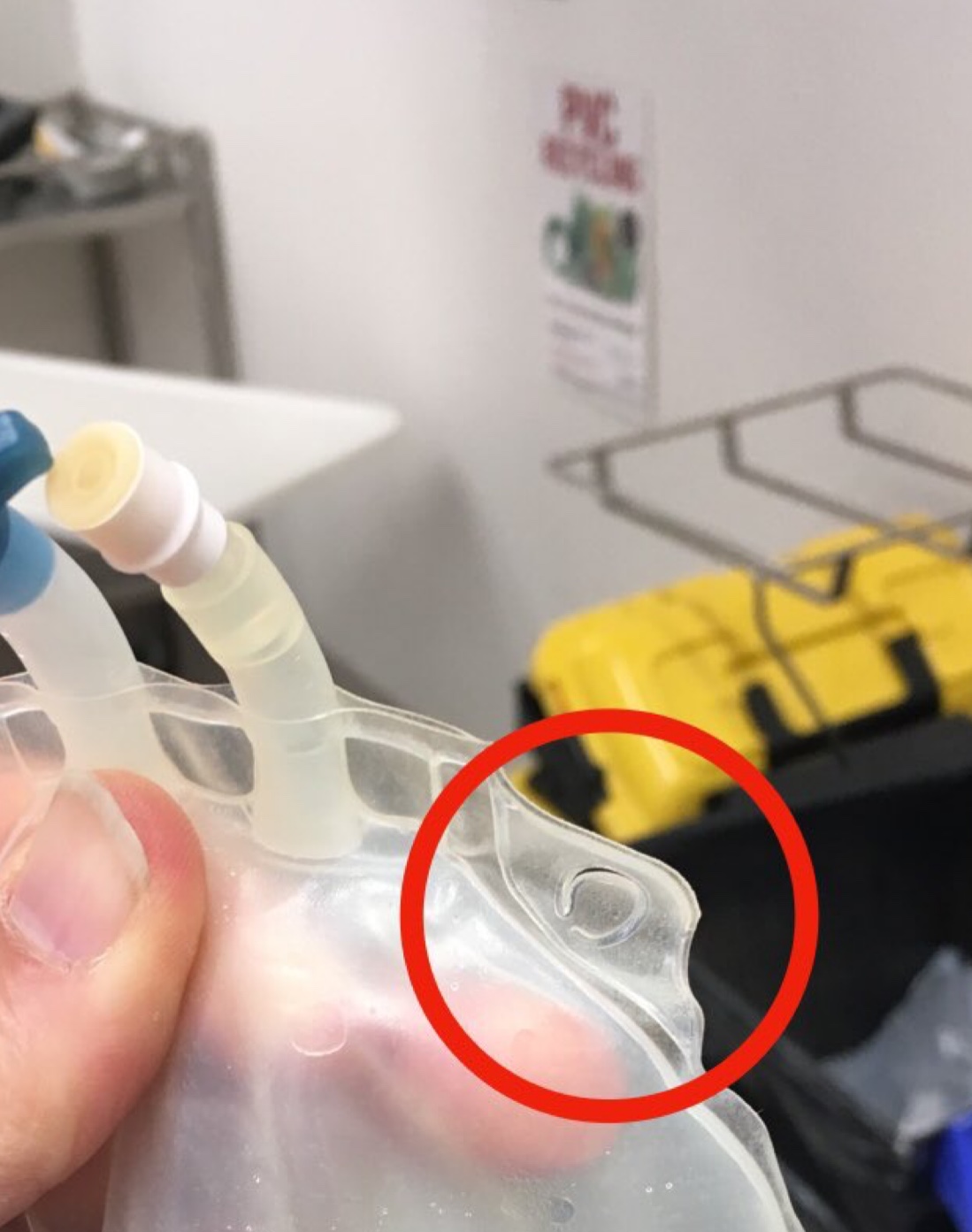
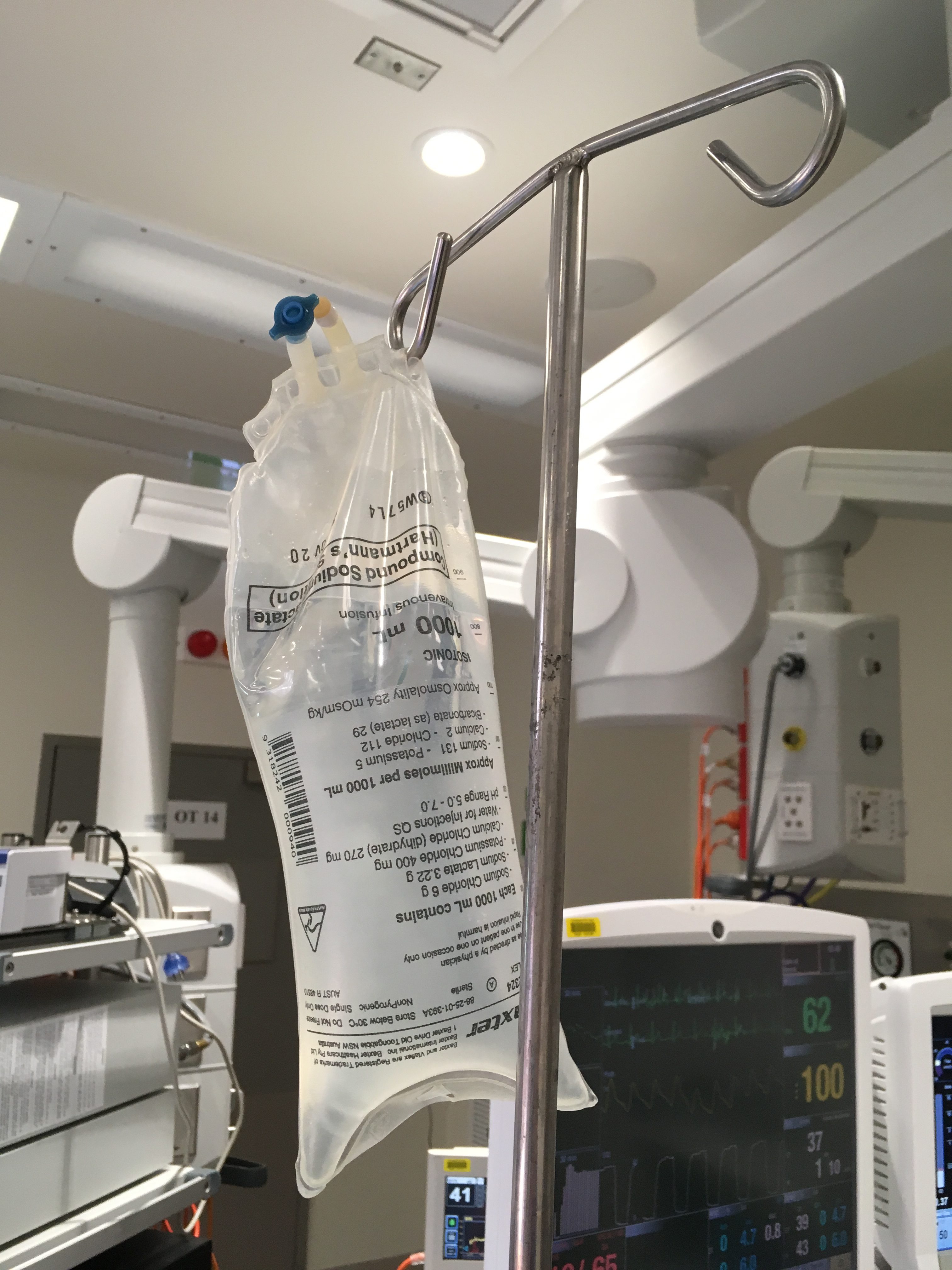
The process of disconnecting a valveless intravenous fluid bag allowed air to enter it. When the bag was later re-connected (re-spiked) the air passed through the intravenous line and into Ruby’s circulation leading to her death.
The coroner’s recommendations focused on outlawing the practice of IV fluid bag re-spiking. You can read the full report (see here).

While we agree the process of IV fluid bag re-spiking should be prohibited we recognise this practice persists throughout the world and are aware of several other deaths which have occurred in the same way. This demonstrates ‘The Gap’ that exists between work as perceived by managers and work as performed by front line staff. This gap is endemic to top down safety approaches.
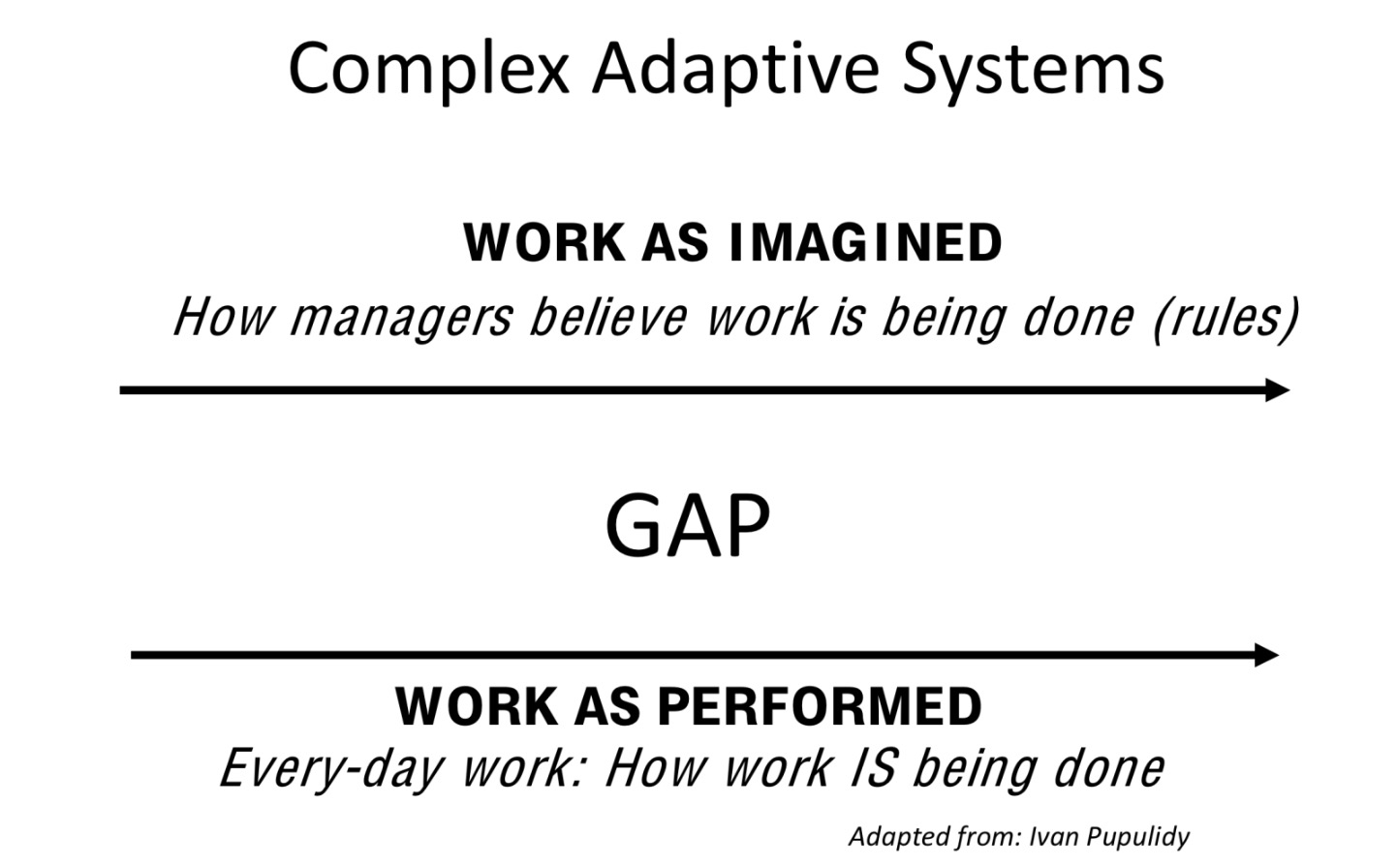
People focussed interventions (education and even point of care reminders to inhibit fluid re-spiking) are relatively less effective safety interventions.
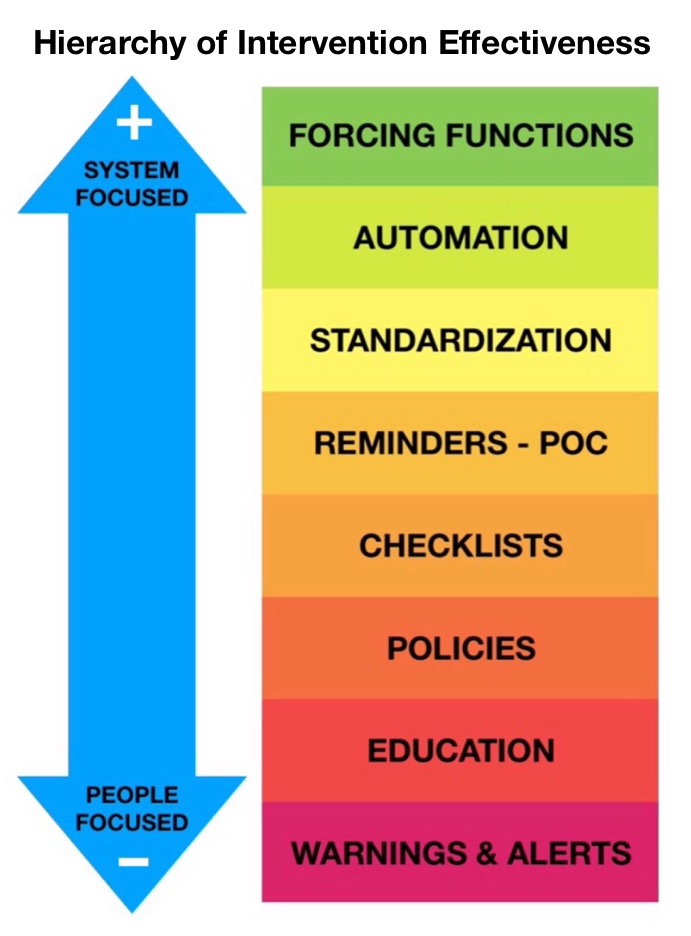
There is a more effective intervention to prevent this problem – valved IV Fluid Bags, which don’t entrain air – they’re already used in many institutions without problem. This represents a forcying function – a design constraint which prevents the adverse event from happening.
Preventing future similar adverse events would require making valved IV fluid bags standard – an intervention we will continue to pursue.
Cost is not an issue here (valved, safer 1L Hartmanns bags from Fresenius Kabi are $1.09 in NSW while valveless bags from Baxter are $1.10). We will save 1c per bag while providing patients with a safer environment.
Progress:
We’ve informed both the Therapeutic And Good Administration (TGA) and the Australian Commission on Safety and Quality in Health Care (ACSQHC).
Help:
If you know of any institution using valveless IV fluid bags (the majority at present) please present this issue to senior hospital managers.
IV fluid bags with valves which prevent air entrainment (on right in image below) are already in use in many hospitals. (Please note air in the top of bag on left). Click on image for video:
Does the design of certain IV fluid bags encourage the practice of re-spiking?
While we have been made aware of several other cases not reported in the literature we will try to maintain a list of cases in the literature here:
Inadvertent venous air embolism during cesarean section: collapsible intravenous fluid bags without self-sealing outlet have risks. Case report
http://www.scielo.br/scielo.php?pid=S0034-70942013000400010&script=sci_arttext&tlng=en
Comments:
Dr Helen Rowlands
SEPTEMBER 16, 2016 AT 9:48 AM EDIT
Should not be hanging bags of fluid in any child. All fluids should go through a syringe driver as happens in UK. This eliminates accidental air entrainment and syringe driver will not push air through.
REPLY
patientsafe
SEPTEMBER 16, 2016 AT 10:56 AM EDIT
Syringe drivers are not always used – particularly in operating theatres. This patient was on an aeroplane retrieval where equipment is limited. We can’t guarantee syringe drivers will always be used.
The risk of air emboli via this mechanism is not limited to the paediatric population and the use of syringe drivers does not contraindicate introducing other safeguards.
REPLY
patientsafe
SEPTEMBER 17, 2016 AT 3:35 AM EDIT
Some transport aircraft may not be large enough to allow syringe drivers or tall enough to allow gravitational IV flow. It’s highly likely the staff member who re-spiked the IV fluid bag wasn’t the person who applied the pressure bag to it. We need to recognise workplace complexity.
Jamie
SEPTEMBER 20, 2016 AT 4:39 AM EDIT
I have worked for three major hospital systems here in the US and all three have very strict no re-spike rules. We use new tubing for every new bag of fluid also. You never reuse bags or tubing.
Maj
SEPTEMBER 18, 2016 AT 1:17 AM EDIT
It would be wise to do a cost-benefit analysis of the (hundreds? thousands? tens of thousands? of) hours of nursing time expended prepping new tubing and spiking a new bag every time. Preparing new tubing could also result in an air embolism if the nurse forgets to flush the line. Depending on how frequently that occurs, forcing nurses away from re-spiking bags could have a small safety benefit, or be even less safe.
Elizabeth Walters-Mabbott
SEPTEMBER 19, 2016 AT 8:56 AM EDIT
I work in intensive care in the UK and our trust has a strict no respike policy, while I can not guarantee this is never done throughout the trust I can be sure it is never done in my department of work for 2 reason the risk of air administration and also it is a high infection control risk, a lot of education and awareness went into stopping this practice and it took some time but it has been the normal to never respike in our trust for a number of years now and it is concerning that this practice still takes place.


2 Comments